1、
On August 4, 2021, Chen Jing, deputy director of the Standing Committee of the Shanghai Municipal People's Congress and director of the Shanghai Hongqiao Business District Management Committee, visited the opening and operation of Yongtai Medical with Yang Jie, chairman of the Shanghai Xinhongqiao International Medical Park, and Hong Weizhong, general manager, introduced the company's application of AI model to establish a dynamic data collection and monitoring scene for cardiac health based on its digital medical strategy under the Royal Life International Group.

2、
On August 7th and 8th, 2021, officials from the Consulate General of the Kingdom of Portugal in Shanghai, Mr. Nuno Miguel Lopes, and Jacob Flowers, co-founder of Smart Shanghai, visited Yuntai Medical to experience the British general practice medical services and health management programs. Currently, Yuntai Medical's British general practice medical services are gradually meeting the high-end medical needs of consulate officials and foreigners in Shanghai.
3、
On August 23, 2021, Dr. Liu, a clinical medicine doctor from Fudan University and the director of the Kidney and Hematology Department at Huashan Hospital, conducted a face-to-face exchange with Yintai's in-house experts and professors on "lipid purification therapy." Lipid abnormalities are now widely recognized as the main factor in cardiovascular and cerebrovascular diseases, and are the culprit behind the arteriosclerosis that affects arteries throughout the body, posing a huge threat to human health. How to effectively intervene and prevent cardiovascular and cerebrovascular diseases was the main topic of discussion at the exchange meeting.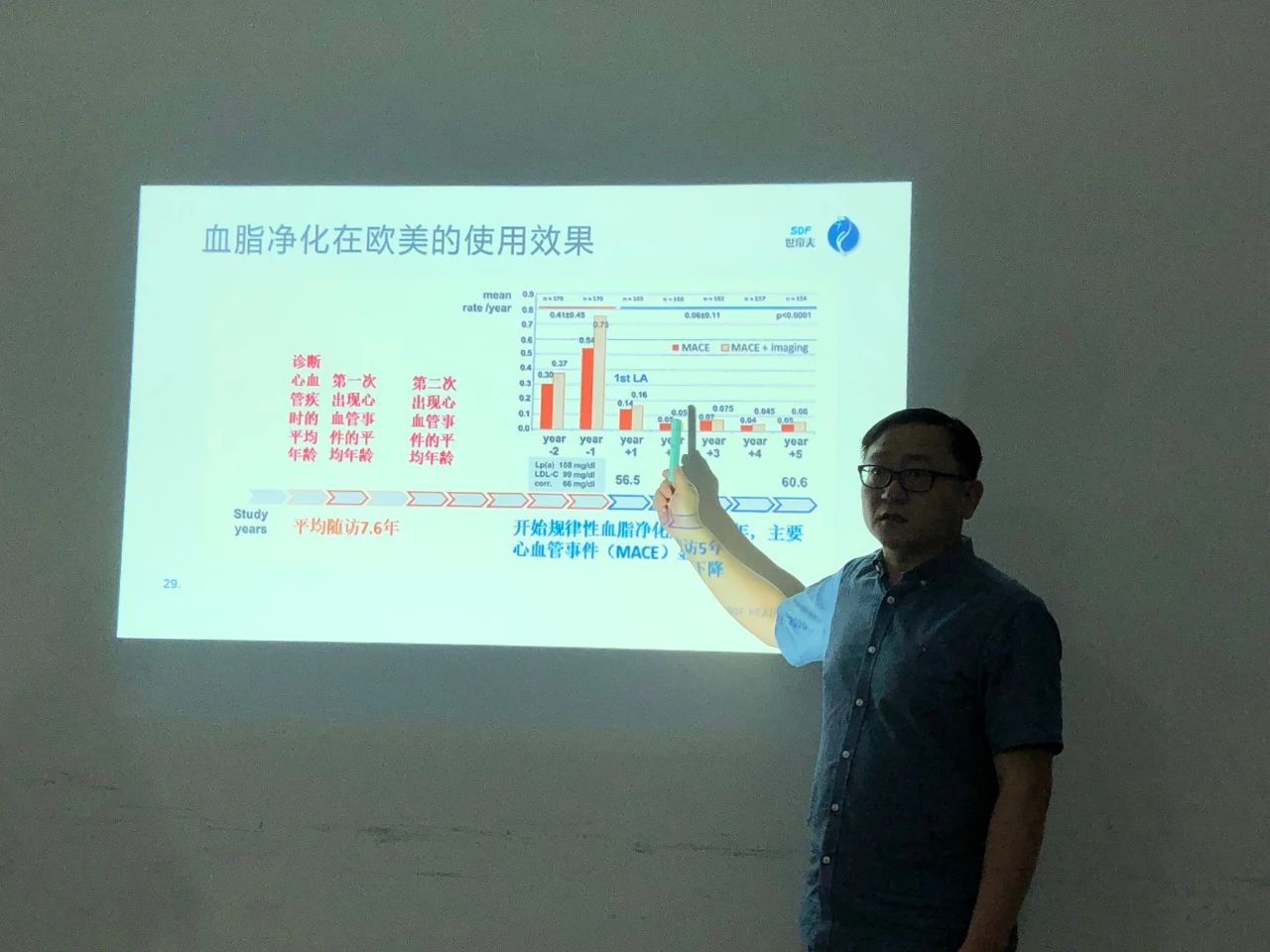
4、
The Jiangsu Provincial Health Commission has issued a document to create a favorable environment for clinical research on immune cell therapy.The Jiangsu Provincial Health Commission has proactively released the "Coordinated Response to the Proposal Submitted by the 12th Standing Committee of the Jiangsu Provincial Political Consultative Conference No. 0813" to the public, which mentions that the Commission will play its role as the regulatory authority for medical and health institutions to coordinate with relevant departments to formulate and issue policies and regulations for the trial and implementation of immune cell therapy fees and entry requirements, as well as to promote synergistic innovation in medical, research, production, and application in Changzhou. The Commission will create a favorable environment for the clinical research of immune cell therapy.


 2024-09-13 10:12:21
2024-09-13 10:12:21